How Not to Poison a Silver Bath

Well, we'd certainly like to avoid a good old fashioned "who done it?" when it comes to the poisoning of a silver bath. As the single most expensive component of the wet plate process, it's worth familiarizing yourself with some of the usual suspects responsible for the unwanted troubles associated with chemical poisoning of silver nitrate.
Suspect #1, Copper
Copper makes a nice prototype example because it's fairly ubiquitous. It can be found in such wide ranging places as plumbing fixtures and pocket change. Not only that, copper can be a sneaky culprit, and disguise itself in a variety of common alloys such as various forms of brass and bronze.
Solving this particular mystery begins with a line-up of the usual suspects in the form of a reactivity series. This is a list of metals ranging from the very reactive potassium and sodium at one extreme to the less reactive silver, gold, and platinum at the other.
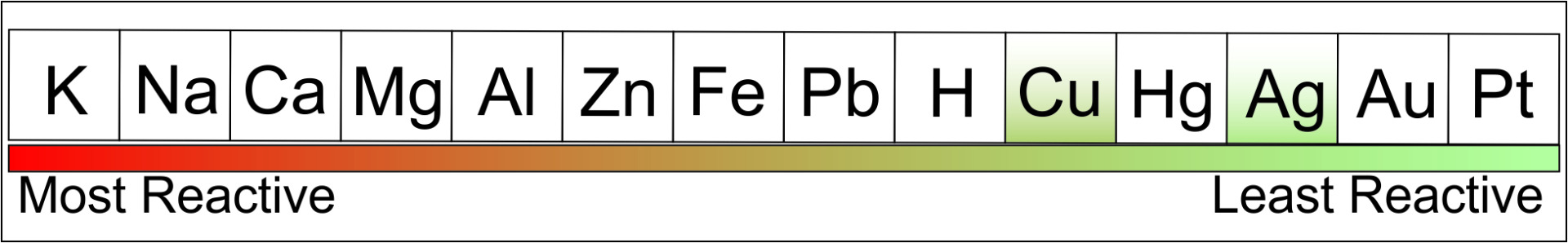
Here you can see that copper is a bit more reactive than silver, meaning, if given the chance, copper will pull the ol' switcheroo and trade places with silver in what's called a single displacement reaction (aka, a redox reaction between metals and their salts).
When dissolved in water, silver nitrate forms an ionic solution of silver. In the presence of copper metal, the silver ion serves as an oxidizing agent which swoops in to pick an electron from the pocket of the unsuspecting copper. Having been "oxidized" in this way (even though there's no oxygen involved in this process, the term still sticks because oxygen is the prototypical thief, and will rip electrons from almost anything it can find) the copper atom becomes a soluble copper ion, which then enters solution. The silver atom which pocketed the electron for itself is "reduced" (in total charge), loses it's status as "ion", and forms silver metal. No longer soluble in water, the silver metal simply precipitates out of solution. The end result of all this electron pick pocketing is an aqueous solution of copper(II) nitrate and a precipitate of metallic silver.
The Devilish Details:
This type of reaction is broadly termed a Reduction Oxidation reaction or Redox. When it occurs between a metal and a metal salt (such as copper and silver nitrate in this case) it's also known as a single displacement reaction, since the two metal bases essentially swap places.
We can write a general representation of this reaction as:
![]()
This reaction occurs if A is more reactive than B. In these reactions the more reactive metal displaces the less reactive metal from its salt.
Placing this in more specific terms, we can write the copper side of the reaction as
![]()
Each silver ion (oxidation state of +1) binds with one electron released by a copper atom.
Writing the silver half of the equation gives us:
![]()
Combining both half equations we get:
![]()
Which simplifies to:
![]()
Here we can see that the copper and silver nitrate swap places with two silver atoms being removed from the solution for every copper atom entering into solution. Along with the nitrate (NO3) that was originally associated with the silver, the result is a solution of copper (II) nitrate (which has a beautifully blue tint).
The BIG Picture:
Now that we've seen a specific example, let's consider the bigger picture. Remember that activity series we saw earlier? Look at all of those nice metals that have a higher activity than silver. All of these will react in the same manner as did copper. And what does this mean in a practical sense for your silver nitrate? If silver nitrate comes into contact with practically any metal, this reaction will occur, and your silver bath will happily swap out the silver for whatever metal it comes in contact with. Leaving you scratching your head wondering "Now, why doesn't the silver bath work?".
If you're spending your time tin typing, you might wonder about your aluminum plates... "Hmmm, why don't these seem to cause a problem if every other metal in the list will?" Here we're saved by an odd twist, in that aluminum is so reactive, it oxidizes almost immediately with oxygen in the air, forming a layer of aluminum oxide on it's surface, which shields it from further reactions. Lucky us!
The take away here is, keep metals of all sorts away from your silver nitrate. Once an unwanted metal is in solution, it's very difficult to remove it again.
But wait! There's more!
Silver Nitrate and Tannins:
Tannins are a class of many different large chemicals composed of carbon, hydrogen, and oxygen. Tannic Acid is a tannin found in tea, and has the formula C76H52O46. Tannins have components called phenols which can be oxidized, and in return reduce silver nitrate, producing nitric acid (HNO3) in the process. The redox reaction for this looks like:
![]()
If you're working with tannic acid or other tannins in some of your other processes, keep this out of your silver bath too!
Okay, now we're done!

